Manipulating Kruppel-Like Factors for RGC Regeneration

About the Research Project
Program
Award Type
Standard
Award Amount
$100,000
Active Dates
April 01, 2010 - March 31, 2012
Grant ID
G2010007
Acknowledgement
Goals
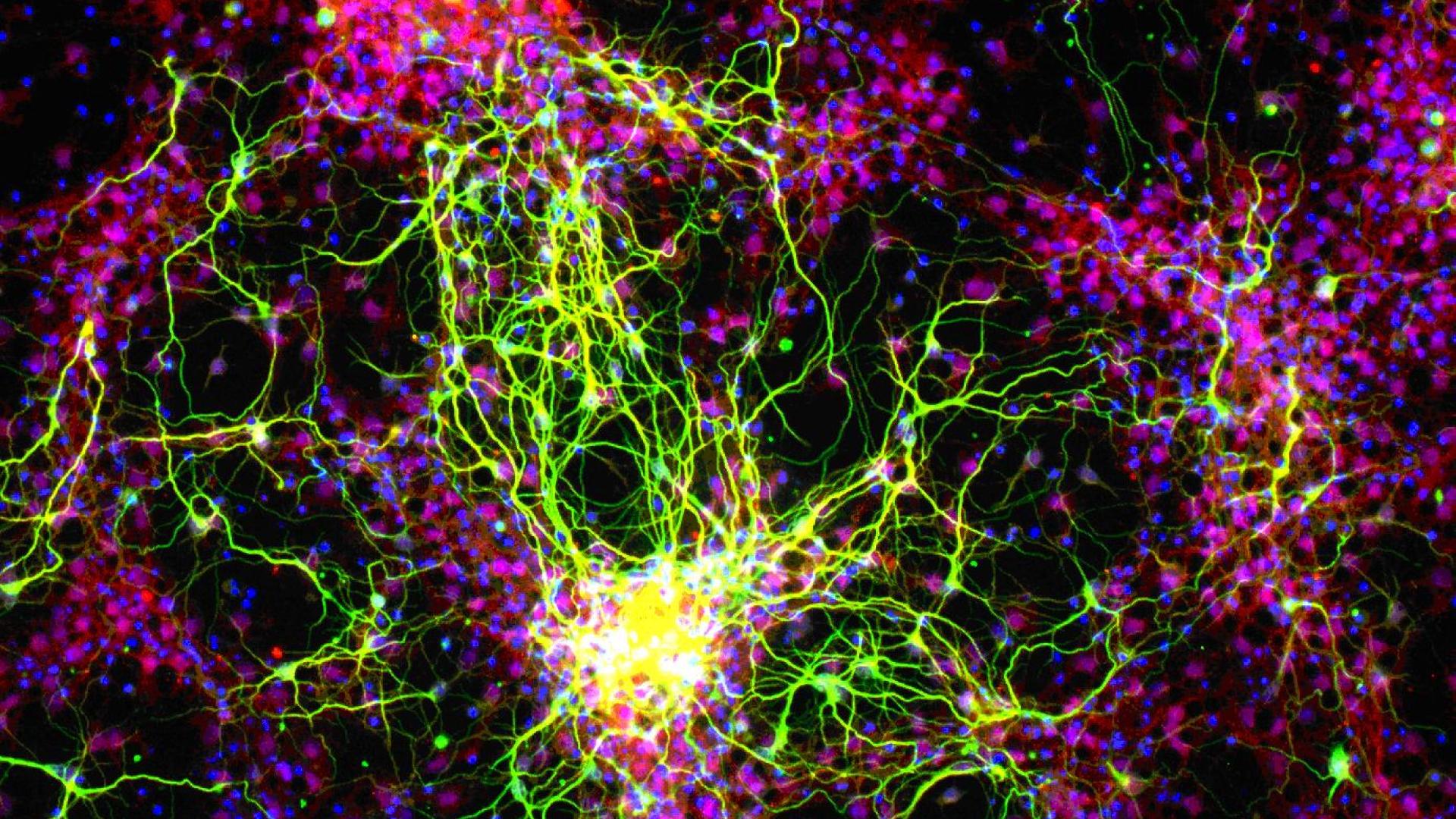
As a result of glaucoma, retinal ganglion cells (RGC) axons are damaged in the optic nerve head, and fail to regenerate through the optic nerve. Here we study methods to enhance RGCs’ intrinsic capacity for optic nerve regeneration through manipulation of the KLF” family of transcription factors using a gene therapy approach. Enhancing optic nerve regeneration may enhance recovery from glaucomatous optic nerve damage.”
Summary
In glaucoma, the optic nerve becomes damaged and fails to regenerate. Because the optic nerve is the only connection between the eye and the brain, this failure of regeneration causes permanent vision loss. We have recently discovered a group of genes called Kruppel-Like Factors (KLFs) that appear to control optic nerve regeneration; this work was published in the journal “Science”. In these experiments, we test whether we can enhance optic nerve regeneration further with gene therapy to manipulate multiple KLFs. In Aim 1 we are building special gene therapy technology that can deliver KLFs to retinal ganglion cells, the neurons in the eye that normally project through the optic nerve. In Aim 2 we test these vectors in an animal model of optic nerve degeneration, to see if they further enhance regeneration. If successful, this will represent a new approach to treating glaucoma and to restoring lost vision.
Jeffrey Goldberg is a physician-scientist who studies the survival and regeneration of retinal ganglion cells in the lab, in addition to seeing patients with glaucoma in his clinical practice.
Progress Updates
Normally, patients with glaucoma can get permanent loss of vision, because there is no regeneration of the optic nerve axons that become damaged in this disease. Dr. Goldberg’s team set out to promote RGC-mediated optic nerve regeneration through manipulation of the KLF family using a gene therapy approach. The goals of this award were accomplished as stated, namely: (1) to develop novel viral vectors capable of delivering specific gene therapy sequences called small hairpin RNAs; and (2) to begin to optimize their use in enhancing optic nerve regeneration in a model of axon injury that mimics certain facets of glaucoma. Specifically, the team created “cassettes” or gene therapy sequences of small hairpin RNAs that target many of the KLFs, and popped these cassettes into adeno-associated viral (AAV) delivery vectors. These gene therapy delivery vectors were packaged into virus that had been engineered to get into cells, but couldn’t cause damage since their infectious “guts” were removed. The team members then injected the packaged virus into the eyes of mice with an optic nerve injury similar to glaucoma. They discovered that some of their anti-KLF gene therapy methods promoted axon regeneration. Therefore, Dr. Goldberg’s team has identified a number of KLF genes that could serve as excellent targets for future therapeutic approaches for treatment of glaucoma.
Related Grants
National Glaucoma Research
Retinal Ganglion Cell Axon Degeneration in a 3D Microfluidic Hydrogel Model
Active Dates
July 01, 2024 - June 30, 2026

Principal Investigator
Shruti Patil, PhD
Current Organization
Indiana University School of Medicine
National Glaucoma Research
The Role of Microtubules in Glaucomatous Schlemm’s Canal Mechanobiology
Active Dates
July 01, 2024 - June 30, 2026

Principal Investigator
Haiyan Li, PhD
Current Organization
Georgia Institute of Technology
National Glaucoma Research
Pressure-Induced Axon Damage and Its Link to Glaucoma-Related Vision Loss
Active Dates
July 01, 2024 - June 30, 2026

Principal Investigator
Bingrui Wang, PhD
Current Organization
University of Pittsburgh