Development of Pharmacological Chaperone Therapy for Inherited Primary and Juvenile Open Angle Glaucoma

About the Research Project
Program
Award Type
Standard
Award Amount
$100,000
Active Dates
April 01, 2008 - March 31, 2011
Grant ID
G2008095
Acknowledgement
Goals
The researchers’ aim is to develop a new therapy for inherited glaucoma, which in many cases is caused by mutations in a protein called myocilin.
Summary
Myocilin forms part of the trabecular extracellular matrix (TEM) in the eye and is important in regulating eye pressure. When the TEM doesn’t function correctly, eye pressure increases, leading to retinal degeneration and vision loss. Human trabecular meshwork (HTM) cells, which produce the matrix, recognize mutations in myocilin and prevent mutant myocilin from being secreted to the TEM. Instead, mutant myocilin remains in the interior of HTM cells, causing the HTM cells to die. This disrupts the TEM, causing increased eye pressure and eventually glaucoma. The team hopes to discover a drug molecule that interacts with mutant myocilin inside the HTM cells to restore secretion of myocilin to the TEM. This could prevent the mutant protein from accumulating in the HTM cells and keep these cells alive. In addition, the TEM could be restored and better able to control eye pressure. This should slow the retinal degeneration associated with glaucoma. The researchers seek to better understand the molecular structure of myocilin, and then to identify and test drug candidates that bind to the myocilin.
Progress Updates
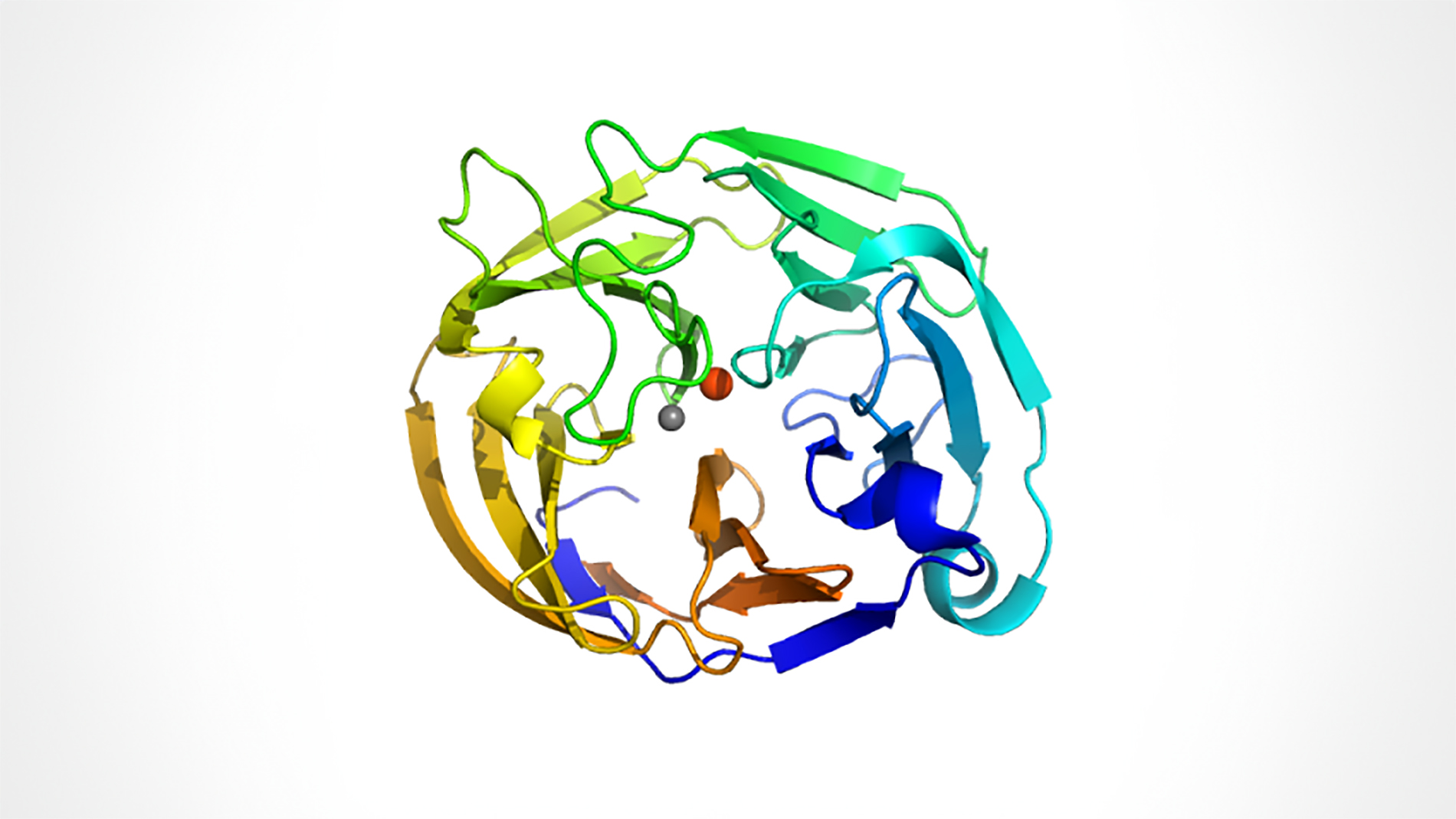
Dr. Raquel Lieberman and collaborators studied the structure and stability of myocilin, the protein most strongly linked to both adult-onset and juvenile inherited Primary Open Angle Glaucoma (POAG). Myocilin protein forms part of a matrix in the eye that is important in regulating eye pressure. Human trabecular meshwork (HTM) cells, which generate the matrix components, recognize mutant myocilin proteins and prevent them from being secreted to the matrix. However, the mutant proteins remain inside HTM cells, causing these cells to die. This results in a disrupted matrix and increased eye pressure, eventually leading to glaucoma.
To understand how mutant myocilin can cause glaucoma, Dr. Lieberman and collaborators first found a way to express and purify large quantities of normal and selected mutant versions of this protein. Next, these researchers discovered the conditions for “crystallization” of these purified proteins into a form that could be used to determine their structures. Now that they have the myocilin crystals, no doubt they will soon determine its three dimensional structure. Finally, they developed a sensitive, high-throughput assay to determine the stability of the different forms of myocilin. Dr. Lieberman will use the information and techniques generated through this award to search for a new glaucoma drug that may restore the secretion of myocilin and save the HTM cells from dying.
Related Grants
National Glaucoma Research
Enhancing Access to Glaucoma Care Using Artificial Intelligence
Active Dates
July 01, 2025 - June 30, 2027

Principal Investigator
Benjamin Xu, MD, PhD
Current Organization
University of Southern California
National Glaucoma Research
Developing a New Glaucoma Treatment That Avoids Daily Drops
Active Dates
July 01, 2025 - June 30, 2027

Principal Investigator
Gavin Roddy, MD, PhD
Current Organization
Mayo Clinic, Rochester
National Glaucoma Research
Human Retinal Regeneration to Cure Glaucoma
Active Dates
July 01, 2025 - June 30, 2027

Principal Investigator
Karl Wahlin, PhD
Current Organization
University of California, San Diego