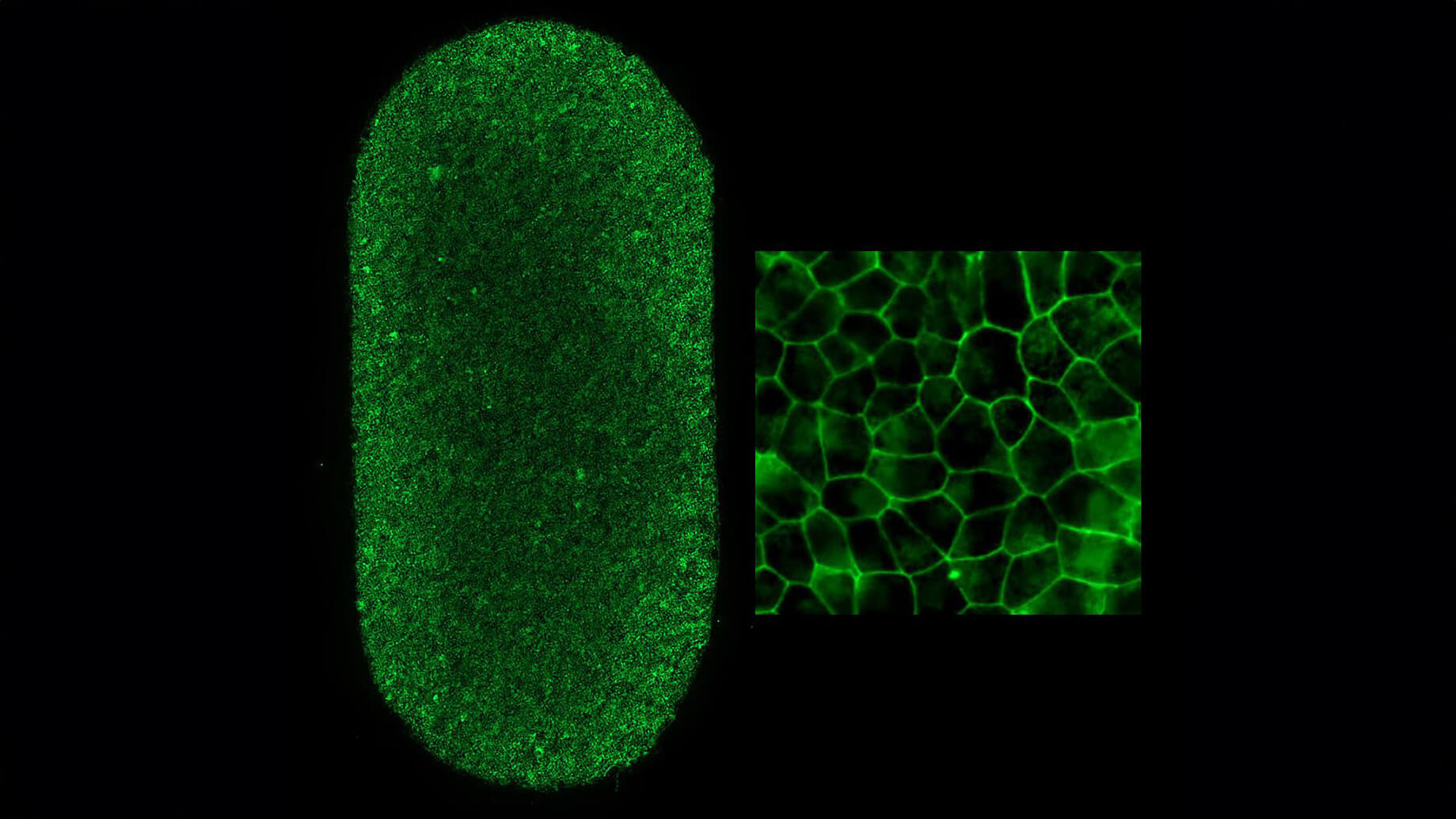
Engineered Eye Tissue Models to Analyze Mechanisms of Age-Related Vision Loss

About the Research Project
Program
Award Type
Standard
Award Amount
$180,000
Active Dates
September 01, 2020 - August 31, 2022
Grant ID
M2020258
Co-Principal Investigator(s)
Eric Nguyen, National Eye Institute
Goals
The role of retinal pigment epithelial cells and retinal blood vessels in the progression of age-related macular degeneration, a disease that leads to progressive vision loss with age, is not fully understood. This research project uses 3D bio-printed human tissue models to clarify the role of retinal blood vessels in initiating and progressing macular degeneration. The completion of this project is expected to determine whether the retinal blood vessels can be effective therapeutic targets for countering macular degeneration and suggest novel therapeutics against the disease.
Summary
The roles of retinal pigment epithelial cells and nearby blood vessels in the progression of age-related macular degeneration (AMD), a disease that leads to progressive vision loss with age, are not fully understood. This research project uses 3D bio-printed human tissue models to clarify the role of retinal blood vessels in initiating and progressing macular degeneration. The completion of this project is expected to determine whether the retinal blood vessels can be effective therapeutic targets for countering macular degeneration and suggest novel therapeutics against the disease. Our work will generate artificial models of the retinal pigment epithelium (RPE) – a supporting cell layer that maintains retinal health – and nearby blood vessels to decode how RPE and blood vessels dictate AMD progression over time. The artificial models of the RPE are designed to allow close examination of communication signals made between RPE and blood vessels. Using these models, we will first simulate disease conditions that are known to initiate and progress AMD, and then isolate which RPE signals cause changes to the blood vessels during simulated AMD. Afterward, we will determine how these changes in blood vessel behavior affect AMD progression. Using this information, we will use genetically engineered induced pluripotent stem cells to create RPE that carry genetic mutations known to increase patient risk of contracting AMD.
Our goal here is to determine if and how these genetic mutations change blood vessel behavior and determine the severity of these effects on AMD progression. Finally, when we have identified key RPE/blood vessel interactions that drive AMD, we will use our artificial tissue models as drug testing platforms. These models will allow for detailed determinations of the effectiveness and toxicity of AMD therapeutics. One of the novelties of our project is that we are focusing on a particular feature of RPE-associated blood vessels that have not been closely studied in the context of AMD: fenestrations. These are blood vessel pores that increase the rate of oxygen and nutrient transfer between blood and RPE and can play a critical role in the progression of AMD. If we determine an observable link between fenestrations and AMD using artificial tissue models and high-resolution measurement methods, fenestrations can serve as a novel therapeutic target for a new line of drugs that may be effective in preserving ocular health. These results will clarify the molecular and genetic mechanisms that drive dry AMD and propose novel therapeutics that slow or prevent AMD.
Related Grants
Macular Degeneration Research
A Blood Test to Measure Genes Associated with Macular Degeneration
Active Dates
July 01, 2025 - June 30, 2028

Principal Investigator
Jerzy Szablowski, PhD
Current Organization
William Marsh Rice University
Macular Degeneration Research
Tracking Biological Responses to Lifestyle Changes in AMD Patients
Active Dates
July 01, 2025 - June 30, 2027

Principal Investigator
Joëlle Vergroesen, PhD
Current Organization
Erasmus University Medical Center Rotterdam (Erasmus MC)
Macular Degeneration Research
Investigating AMD-Like Disease in Animal Models
Active Dates
July 01, 2024 - June 30, 2027

Principal Investigator
Brittany Carr, PhD
Current Organization
University of Alberta