Neuroregenerative Strategies in Glaucoma

About the Research Project
Program
Award Type
Bold Ideas initiatives
Award Amount
$1,200,000
Active Dates
July 01, 2015 - December 31, 2019
Grant ID
C2015201
Goals
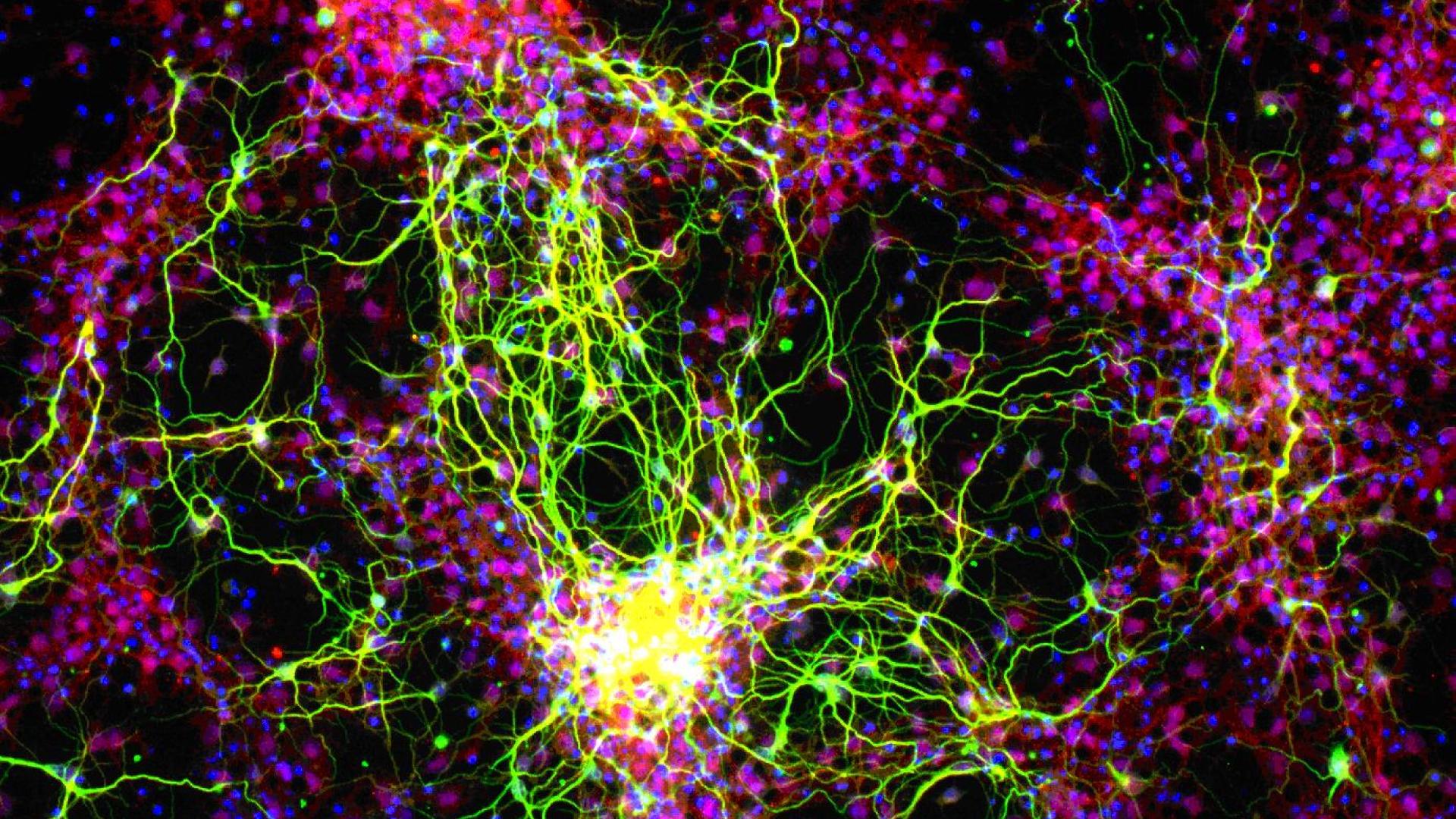
For glaucoma, the goal is to protect sight by preserving the function of retinal ganglion cells (RGCs), which make up the optic nerve. In many forms of glaucoma, damage associated with increased intraocular pressure (IOP) begins to damage the optic nerve, ultimately killing the RGCs, with subsequent associated vision loss. Dr. Goldberg is conducting a phase 2 clinical trial where he will implant into the eye a tiny device, called NT-501 encapsulated cell therapy (NT-501 ECT). The NT-501 ECT contains cells designed to deliver a steady stream of a growth factor, called ciliary neurotrophic factor (CNTF), to test whether it can protect damage to the optic nerve, and, possibly, enhance visual function in patients with glaucoma.
In parallel, we have advanced the field of stem cell-to-retinal ganglion cell differentiation, discovering a new pathway regulating the generation of RGCs from human stem cells, and discovering that human stem cells help support the survival of RGCs after transplant.
Summary
NCT02862938 is the identifying number for this two year phase 2 clinical trial, with the goal to enroll 60 participants, age 18 or older, with clinically stable glaucoma in one or both eyes. Participants will be randomly assigned to either a treatment group (“subjects”) or a non-treatment group (“controls”).
Subjects will have the NT-501 ECT investigational product implanted in one eye, and will be followed for 24 months. Controls will not receive an implant but will be followed in a similar manner. At a later point, controls are expected to have the option to receive the NT-501 ECT treatment.
Patients will be evaluated quarterly over 2 years. The main outcome measure being used to judge the treatments effectiveness is change in visual field (side/peripheral vision) at 6 months, although additional outcomes will be evaluated over the course of the two-year trial. Some of these secondary outcome measurements look at retinal nerve fiber layer thickness, contrast sensitivity (difference in luminance or color that makes an object distinguishable), best corrected visual acuity (the clarity or sharpness of vision), visual field pattern standard deviation, and structural changes in the optic nerve head (the point of exit for RGCs leaving the eye).
For more information about this clinical trial, please visit:
Related Grants
National Glaucoma Research
Understanding How Variants in LOXL1 Affect Pseudoexfoliation Glaucoma Risk
Active Dates
July 01, 2024 - June 30, 2026

Principal Investigator
Hannah Youngblood, PhD
Current Organization
Georgia Institute of Technology
National Glaucoma Research
Retinal Ganglion Cell Axon Degeneration in a 3D Microfluidic Hydrogel Model
Active Dates
July 01, 2024 - June 30, 2026

Principal Investigator
Shruti Patil, PhD
Current Organization
Indiana University School of Medicine
National Glaucoma Research
The Role of Microtubules in Glaucomatous Schlemm’s Canal Mechanobiology
Active Dates
July 01, 2024 - June 30, 2026

Principal Investigator
Haiyan Li, PhD
Current Organization
Georgia Institute of Technology